
Ambu Inc., a rapidly growing medical device maker and pioneer of sterile, single-use endoscopes, announced today that the Ambu® aScope™ Duodeno has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
“At Ambu, we are determined to advance patient safety through innovative design of single-use devices, and we are excited to improve safety for the 2 million patients each year who require an ERCP (Endoscopic Retrograde Cholangio-Pancreatography) procedure,” said Juan Jose Gonzalez, CEO of Ambu A/S, which is based in Copenhagen, Denmark. “It’s no longer necessary to balance the necessity of the procedure against the risk of infection from a reusable endoscope. Now, both doctor and patient can focus on diagnosis and treatment by using a sterile, single-use duodenoscope.”
Reusable duodenoscopes have been under increasing critique from the FDA in recent years because there have been cases of device-related infections and patient fatalities. In August 2019, the FDA recommended duodenoscope manufacturers and healthcare facilities transition to duodenoscopes that are partially or completely single-use.
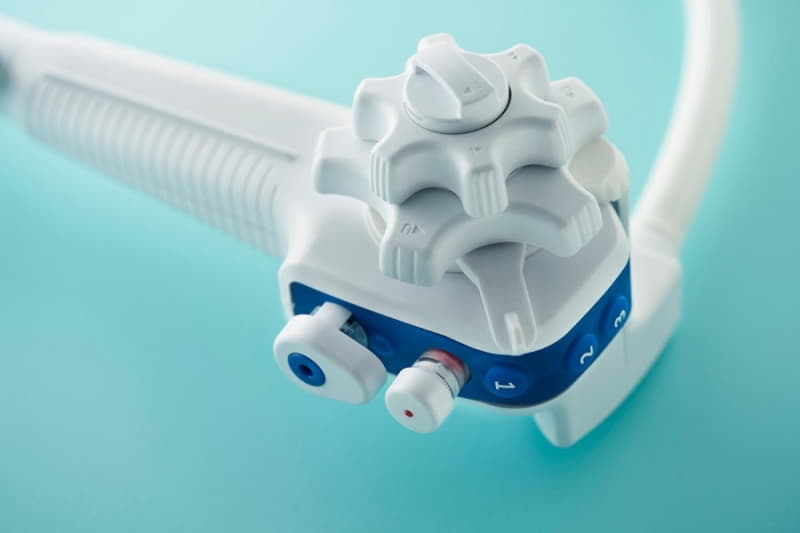
“The aScope Duodeno is sterile, single-use and seamlessly integrates into existing hospital systems and offers an intuitive, lightweight design with similar functionality to reusable duodenoscopes,” said Jens Kemp, Vice President of Marketing at Ambu Inc. “Over the past six months, we have expanded our sales organization and built a dedicated commercial infrastructure for gastroenterology. After today’s FDA clearance, we will now approach our customers to arrange product demonstrations, set up evaluations and promote what Ambu has to offer within the field of GI.”
A single-use duodenoscope system
Ambu’s duodenoscope solution consists of a single-use endoscope – Ambu® aScope™ Duodeno – and a reusable processor unit – Ambu® aBox™ Duodeno. The FDA clearance covers both devices.
Duodenoscopes are used for visual examinations of the duodenum and play a key role in the diagnosis and treatment of conditions like gallstones, pancreatitis, tumors or cancer in the bile ducts or the pancreas. In the U.S., there are an estimated 600,000 procedures using duodenoscopes conducted annually.
New payment to promote patient access
Payers are already recognizing the value of single-use duodenoscopes. The Centers for Medicare & Medicaid Services (CMS) recently announced a new Transitional Pass-Through (TPT) payment category for single-use endoscopes used in outpatient ERCP procedures. With this decision, CMS recognizes that single-use duodenoscopes are essential to improving patient safety. The aScope Duodeno is eligible for the TPT payment category.
Initiating Post-market study
Having obtained FDA clearance, Ambu will initiate a 500-patient post-market study at multiple centers in the U.S. as planned. A sub-set of the study will be made public during Q1 of Ambu’s financial year 2020/21 when data from a minimum of 60 procedures is available.
Robust innovation product pipeline
Over the next three years, Ambu is introducing 15 new devices across all major areas of endoscopy. This includes three additional devices specifically for GI specialists: a colonoscope, a gastroscope and a cholangioscope.
Built on a decade of experience
Ambu launched the world’s first single-use flexible bronchoscope, the Ambu® aScope in 2009. Ten years later, in 2019, over 600,000 Ambu single-use endoscopes were used in more than 6,000 hospitals making Ambu the world’s largest supplier of single-use endoscopes. The aScope Duodeno was designed with input from a global gastroenterologist consultant board along with engineering expertise from the team of Invendo Medical acquired by Ambu in 2017.


